A feat of basic neuroscience co-led by UNC School of Medicine scientists, the discovery of a set of arousal-related neurons could help scientists develop better treatments for anxiety disorders, psychiatric illnesses.
Strong emotions such as fear and anxiety tend to be accompanied and reinforced by measurable bodily changes including increased blood pressure, heart rate and respiration, and dilation of the eyes’ pupils. These so-called “physiological arousal responses” are often abnormally high or low in psychiatric illnesses such as anxiety disorders and depression. Now scientists at the UNC School of Medicine have identified a population of brain cells whose activity appears to drive such arousal responses.
The scientists, whose study is published in Cell Reports, found that artificially forcing the activity of these brain cells in mice produced an arousal response in the form of dilated pupils and faster heart rate, and worsened anxiety-like behaviors.
The finding helps illuminate the neural roots of emotions, and point to the possibility that the human-brain counterpart of the newly identified population of arousal-related neurons might be a target of future treatments for anxiety disorders and other illnesses involving abnormal arousal responses.
“Focusing on arousal responses might offer a new way to intervene in psychiatric disorders,” said first author Jose Rodríguez-Romaguera, PhD, assistant professor in the UNC Department of Psychiatry and member of the UNC Neuroscience Center, and co-director of the Carolina Stress Initiative at the UNC School of Medicine.
Rodríguez-Romaguera and co-first author Randall Ung, PhD, an MD-PhD student and adjunct assistant professor in the Department of Psychiatry, led this study when they were members of the UNC laboratory of Garret Stuber, PhD, who is now at the University of Washington.
“This work not only identifies a new population of neurons implicated in arousal and anxiety, but also opens the door for future experiments to systematically examine how molecularly defined cell types contribute to complex emotional and physiological states,” Stuber said. “This will be critical going forward for developing new treatments for neuropsychiatric disorders.”
Anxiety disorders, depression, and other disorders featuring abnormally high or low arousal responses affect a large fraction of the human population, including tens of millions of adults in the United States alone. Treatments may alleviate symptoms, but many have adverse side effects, and the root causes of these disorders generally remain obscure.
Untangling these roots amid the complexity of the brain has been an enormous challenge, one that laboratory technology has only recently begun to surmount.
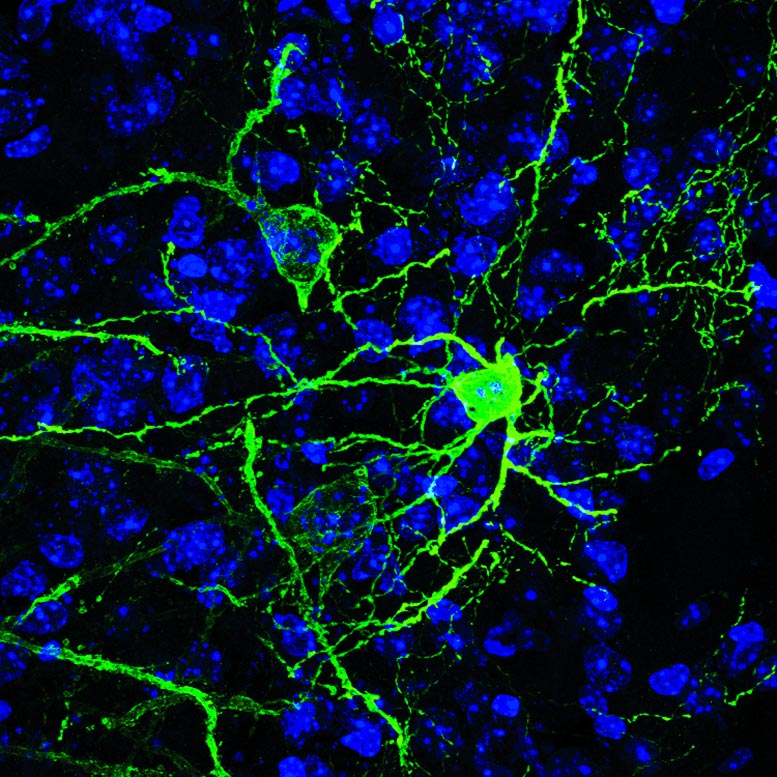
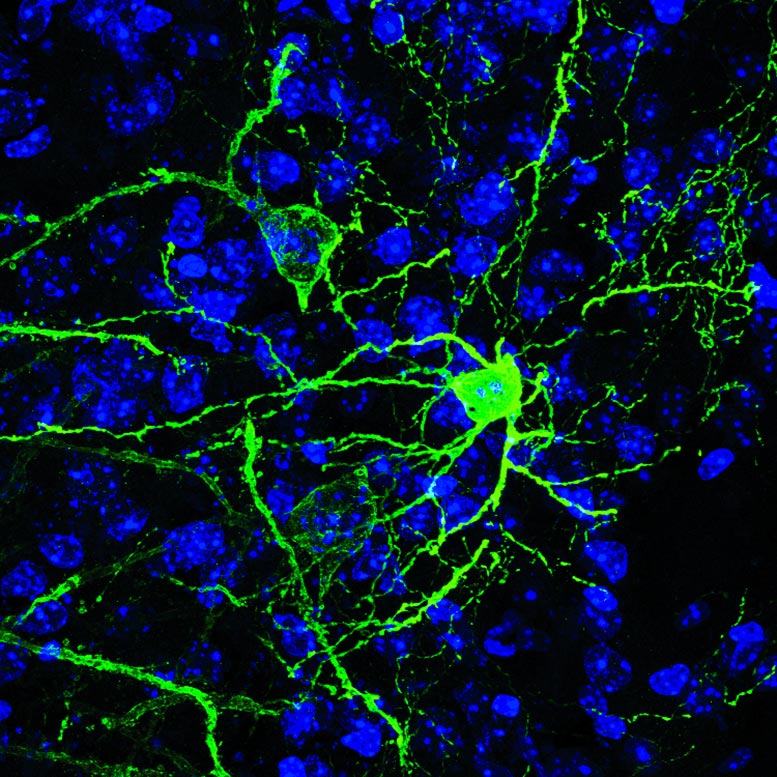
Rodríguez-Romaguera, Ung, Stuber and colleagues examined a brain region within the amygdala called the BNST (bed nucleus of the stria terminalis), which has been linked in prior research to fear and anxiety-like behaviors in mice. Increasingly, scientists view this region as a promising target for future psychiatric drugs. In this case, the researchers zeroed in on a set of BNST neurons that express a neurotransmitter gene, Pnoc, known to be linked to pain sensitivity and more recently to motivation.
The team used a relatively new technique called two-photon microscopy to directly image BNST Pnoc neurons in the brains of mice while the mice were presented with noxious or appealing odors – stimuli that reliably induce fear/anxiety and reward behaviors, respectively, along with the appropriate arousal responses. In this way, the scientists found that activity in these neurons tended to be accompanied by the rapid dilation of the pupils of the mice when the animals were presented with either of these odor stimuli.
The researchers then used another advanced technique called optogenetics – using light to control genetically engineered cells – to artificially drive the activity of the BNST Pnoc neurons. They found that spurring on BNST Pnoc activity triggered a pupillary response, as well as increased heart rate. Optogenetically driving the neurons while the mice underwent an anxiety-inducing maze test (traditionally used to assess anxiety drugs) increased the animals’ signs of anxiety, while optogenetically quieting the neurons had the opposite effect.
“Essentially we found that activating these BNST Pnoc neurons drives arousal responses and worsens anxiety-like states,” Rodríguez-Romaguera said.
The discovery is mainly a feat of basic neuroscience. But it also suggests that targeting arousal-driving neurons such as BNST Pnoc neurons with future drugs might be a good way to reduce abnormally strong responses to negative stimuli in anxiety disorders, for example, and to boost abnormally weak responses to positive stimuli in depression.
The study uncovered evidence that BNST Pnoc neurons are not all the same but differ in their responses to positive or negative stimuli, and the researchers are now cataloguing these BNST Pnoc neuron sub-groups.
“Even this small part of the amygdala is a complex system with different types of neurons,” Ung said. Teasing this apart will help us understand better how this system works.”
Reference: “Prepronociceptin-Expressing Neurons in the Extended Amygdala Encode and Promote Rapid Arousal Responses to Motivationally Salient Stimuli” by Jose Rodriguez-Romaguera, Randall L. Ung, Hiroshi Nomura, James M. Otis, Marcus L. Basiri, Vijay M.K. Namboodiri, Xueqi Zhu, J. Elliott Robinson, Hanna E. van den Munkhof, Jenna A. McHenry, Louisa E.H. Eckman, Oksana Kosyk, Thomas C. Jhou, Thomas L. Kash, Michael R. Bruchas and Garret D. Stuber, 10 November 2020, Cell Reports.
DOI: 10.1016/j.celrep.2020.108362
The other co-authors of the study were Hiroshi Nomura, James Otis, Marcus Basiri, Vijay Namboodiri, Xueqi Zhu, Elliott Robinson, Hanna van den Munkhof, Jenna McHenry, Louisa Eckman, Oksana Kosyk, Thomas Jhou, Thomas Kash, and Michael Bruchas.
The research was supported by the National Institute of Mental Health (F32-MH113327, F30-MH115693, K99-MH118422, T32-MH093315, K99-MH115165, R01-MH112355), the National Institute of Neurological Disorders and Stroke (T32-NS007431), the National Heart, Lung, and Blood Institute (R01-HL150836), the National Institute on Drug Abuse (F32-DA041184, R37-DA032750 & R01-DA038168), the Children’s Tumor Foundation, the Brain and Behavior Research Foundation, and the Yang Biomedical Scholars Award.